

The commonly used excitation source for XPS experiments is monochromated Al Kα where E x = 0.25 eV. Where E m is the measured peak width, E x is the width of the photon source, E lw is the natural line with of the electronic state being measured and E inst is the instrument resolution. If we assume that the peak shape is the convolution of several Gaussian peaks, the measured peak width is given by:Į m 2 = E x 2 + E lw 2 + E inst 2 equation 1 The easiest way to answer this is to understand what contributes to the measured peak width in an XPS spectrum. What affects the energy resolution of my photoelectron spectrometer? So, to properly define the performance of an XPS instrument it is important to know the count rate at a given energy resolution. As the energy resolution increases, the amount of signal collected decreases. And therein lies the paradox of most spectroscopic techniques. Importantly, it doesn’t provide information on the peak intensity (counts per second). It also provides insight into the ability of the spectrometer to resolve, or separate, two closely spaced peaks in a photoelectron spectrum.
It allows the ultimate energy resolution of instruments from different manufacturers to be compared. The longer answer is that the FWHM of the Ag 3d 5/2 peak is a useful indication performance.
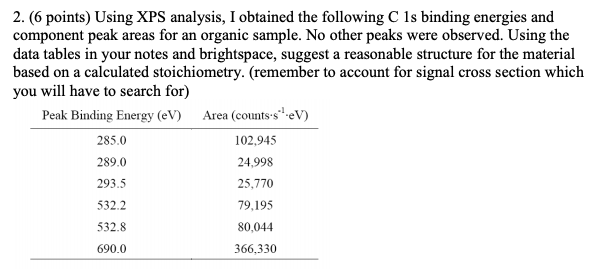
#XPS PEAK ANALYSIS FULL#
Energy resolution of an XPS instrument is usually measured as the full width at half-maximum (FWHM) of the Ag 3d 5/2 peak. XPS is a spectroscopic technique and therefore the energy resolution of the instrument is of fundamental importance.

The chemical or bonding information of the element is derived from these chemical shifts. The BE is related to the measured photoelectron KE by the simple equation BE = hν - KE where hv is the photon (x-ray) energy. The chemical environment of an atom alters the binding energy (BE) of a photoelectron which results in a change in the measured kinetic energy (KE). The importance of surfaces in materials science is discussed in greater detail elsewhere These elastiaclly scattered photoelectrons contribute to the photoelectron peak, whilst photoelectrons that have been inelastically scattered, losing some kinetic energy before leaving the material, will contribute to the spectral background. The surface sensitivity of XPS is determined by the distance that that photoelectron can travel through the material without losing any kinteic energy. The position and intensity of the peaks in an energy spectrum provide the desired chemical state and quantitative information. The kinetic energy of these emitted electrons is characteristic of the element from which the photoelectron originated. These ejected electrons are known as photoelectrons. It is a relatively simple technique where the sample is illuminated with X-rays which have enough energy to eject an electron from the atom. X-ray photoelectron spectroscopy (XPS), also known as ESCA (electron spectroscopy for chemical analysis) is a surface analysis technique which provides both elemental and chemical state information virtually without restriction on the type of material which can be analysed.


 0 kommentar(er)
0 kommentar(er)
